|
|
ISO 9001-2015 4.3 QMS scopeSend comments on this topic |
FREE QHSE Software Click <HERE> to Learn More |
||
QHSE Support >(Site Map) Quality Guidance > ISO 9001-2015 Clauses > ISO 9001-2015 clause 4 >
ISO 9001:2015 Clause 4.3 Determining the scope of the quality management system
|
PLAN |
DO |
CHECK |
ACT |
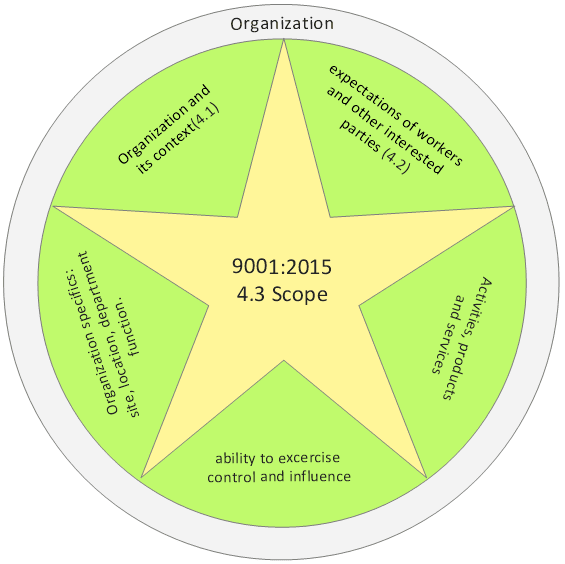
Clause 4.3 Breakdown
4.3 Determining the scope of the quality management system
In regards to QMS “…What do we control, what can we influence…”
The scope should not be used to exclude parts of the organization which may struggle to achieve accreditation. The organization should also not use clause 4.3 to banish those activities, products, services or functions that have the potential to impact on QMS performance, or are likely to place legal or contractual requirements outside of the accredited quality management system. The standard makes no specific mention of “Exclusions,” and uses the term "Applicability" (Annex A.5) when references the scope of an organization's quality management system.
Before an organization finalizes the scope of its quality management system, it makes sense that the requirements of clause 4.1 ‘Understanding the organization and its context’, and clause 4.2 ‘understanding the needs and expectations of interested parties’ have been established and utilised in the evolution of the scope.
The standard itself states:
when determining this scope, the organization shall consider;
a) the internal and external issues referred to in 4.1
b) the requirements of relevant interested parties referred to in 4.2
c) the products and services of the organization
The scope of the organizations quality management system should include activities that impact on quality performance that are under its control or influence. That said, the organization may decide to limit the scope of its quality management system to a specific site, location, department or function. Typically it maybe that the organization is running a pilot project on a smaller scale before attempting wider accreditation: In this case the rest of the organization would be considered an external provider or interested party.
It should be noted that clause 4.3 is required to be kept as documented information, in whichever format the organization deems suitable: e.g. paperwork, digital file, visual representation (flowchart, process-map, mind-map). Whichever format is used, it needs to be made available to interested parties.
Should the organisation consider some clauses of the standard are outside the scope of their quality management system i.e. not applicable, the following is an example of how this maybe addressed within the documented information required for the QMS scope.
•Clause 8.3 – design and development of products and services
the organisation does not undertake design and development work.
•Clause 7.1.5.2 – measurement traceability
the organisation does not provide any products or services where measurement traceability is a requirement.
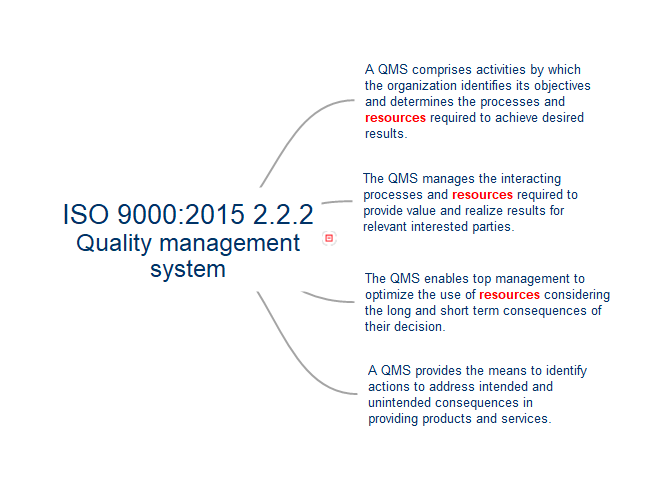
Mind map for the requirements and resource considerations needed for a Quality Management System as defined by ISO 9000:2015 for use in ISO 9001:2015
Useful integrated management system cross references
ISO 14001-2015 4.3 - Determining the scope of the environmental management system
ISO 45001-2018 4.3 - Determining the scope of the OH&S management system
Help file v2.276.407 : QHSE Support - Website On Safe Lines
onsafelines.com QHSE Software 2025 : Webmaster: Brian G. Welch MSc(QHSE), NVQ4(OH&S), CMIOSH