|
|
ISO 9001-2015 7.5 Documented informationSend comments on this topic |
FREE QHSE Software Click <HERE> to Learn More |
||
QHSE Support >(Site Map) Quality Guidance > ISO 9001-2015 Clauses > ISO 9001-2015 clause 7 >
ISO 9001:2015 Clause 7.5 Documented Information
|
PLAN |
DO |
CHECK |
ACT |
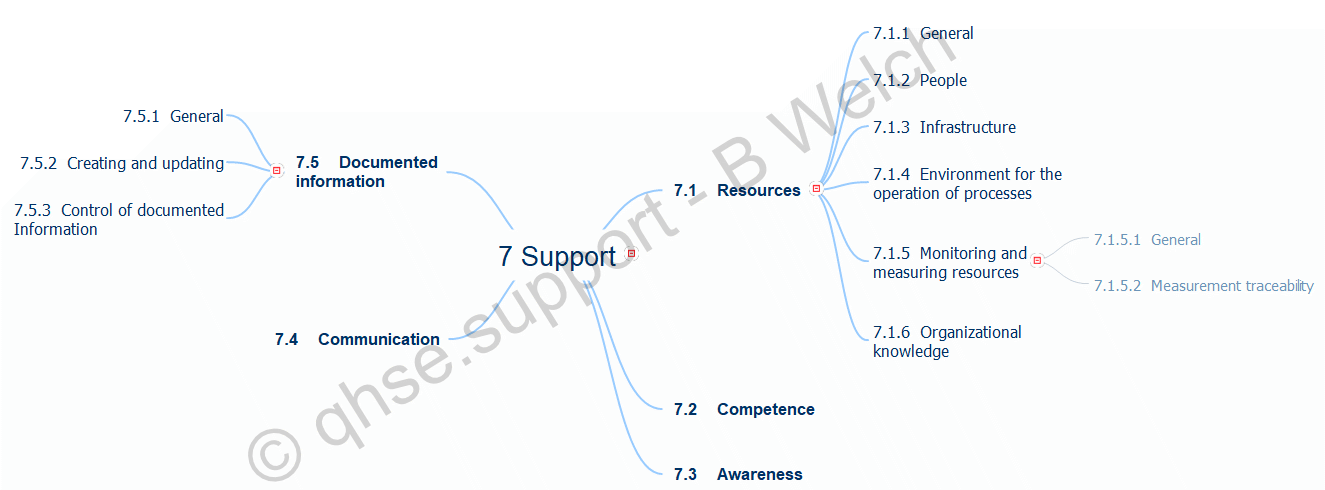
ISO 9001:2015 Clause 7 mind map
Clause 7.5 Breakdown
7.5 Documented information
7.5.1 General
7.5.2 Creating and updating
7.5.3 Control of documented Information
The clearest change here is that there is no equivalent to clause ISO 9001-2008 4.2.2 Quality Manual within this clause. Many organisation will have placed a lot of time and effort in the production of their quality manual and be rightly very proud of this top tier document (others may have created a document merely to assist in keeping auditors happy..). However, you will probably find that your Quality Manual will help you meet the requirements of ISO 9001-2015 clause 4.3 'system scope' and 4.4 'system process'. You should think of the Quality Manual as a document stating requirements i.e the specification for the quality management system for the organisation. The quality manual is still to be managed under the requirements clause 7.5 documented information, it's merely that no medium is given a specific clause... Note: quality manuals can vary in detail and format to suit the size and complexity of an individual organisation.
Within ISO 9000-2015 a document is defined as "information (meaningful data) and the medium on which it is contained"; whereas document information is defined as "information (meaningful data) required to be controlled and maintained by an organisation". Note: Records are also classed as document (meaningful data) stating the results achieved or providing evidence of activities performed.
Neither of these two definitions contain the word document. This is probably because documents are still very much thought of as physical entities that one can hold in ones hand. The use of the word document is too restrictive, however, the term medium more readily covers today's technologies such us paper, magnetic, electronic, computer storage devices, digital and standard photography i.e anything capable of holding meaningful data. Examples being an organisation intranet or designated controlled workstations. Employees, customer and or selected regulatory authorities can be given access to the quality management system documented information upon request.
The standard also makes it clear that the extent of documented information for a quality management system can differ from one organisation to another due to:
•the size of organisation and its type of activities, processes, products and services;
•the complexity of processes and their interactions;
•the competence of persons. (ability to apply knowledge and skills to achieve intended results)
Within ISO 9000-2015 a 'quality management system' is defined as part of the management system with regards to quality. Clearly other managements such as occupational health and safety and environmental management will have elements that will overlap.
continued on next page...
Useful integrated management system cross references
ISO 14001
•ISO 14001-2015 7.5 Documented information
ISO 45001
•ISO 45001-2018 7.5 Documented information
Help file v2.276.407 : QHSE Support - Website On Safe Lines
onsafelines.com QHSE Software 2025 : Webmaster: Brian G. Welch MSc(QHSE), NVQ4(OH&S), CMIOSH