|
|
ISO 9001-2015 7.5 Overview p1Send comments on this topic |
FREE QHSE Software Click <HERE> to Learn More |
||
QHSE Support >(Site Map) Quality Guidance > ISO 9001-2015 Clauses > ISO 9001-2015 clause 7 >
ISO 9001:2015 Clause 7.5 Documented Information page 1 of 3
|
PLAN |
DO |
CHECK |
ACT |
Clause 7.5 Overview
approve documented information adequacy prior to issue.
The key question here is what is mean by adequacy? With regards to the control of documented information it is reasonable to extrapolate that the documented information needs to be fit for purpose and people with adequate knowledge and authority have reached a concord on its contents and impact prior to any authorised issue. This normally involves some level of signatory acceptance, for example a three level approval process might require the following signatures.
• Process Developed / Documented By
• Process Owned By
• Process Authorised By
review and update as necessary and re-approve documented information.
It is in the interest of all parties, that a concord on any changes is passed through a similar process as to the original documented information. Failure to identify key process changes will without doubt impact on the quality management system effectiveness and introduce variation where no variation should exist. To the most part the documented information owner should initiate the review process, it makes sense that the initial approval signatories complete the review, update and re-approval, however, frequently these employees will no longer be available, so the system should be robust enough to cater for such situations without adverse effect on the processes being changed.
ensure that changes and the current revision status of documents are identified.
On the face of it, this would seem a fairly straightforward requirement, however, the clause does not specify how you achieve this requirement or make it clear if its the change of status or the nature of change that should be identified. Logically thinking clause 7.5.2 is more to do with the identification. The benefits are clear and it makes life so much easier to have amendments clearly identified, but how is this to be achieved and communicated. Control logs, documented information issue and revision numbers (on all pages), amendments page, highlighted text, struck-through text, lines down the left or right side of changed text, memo's or e-mails to documented information holders, etc etc are just some examples.
Point of note: deleted text is more difficult to identify and you need to consider how to achieve this if its removal could affect the process if not communicated successfully.
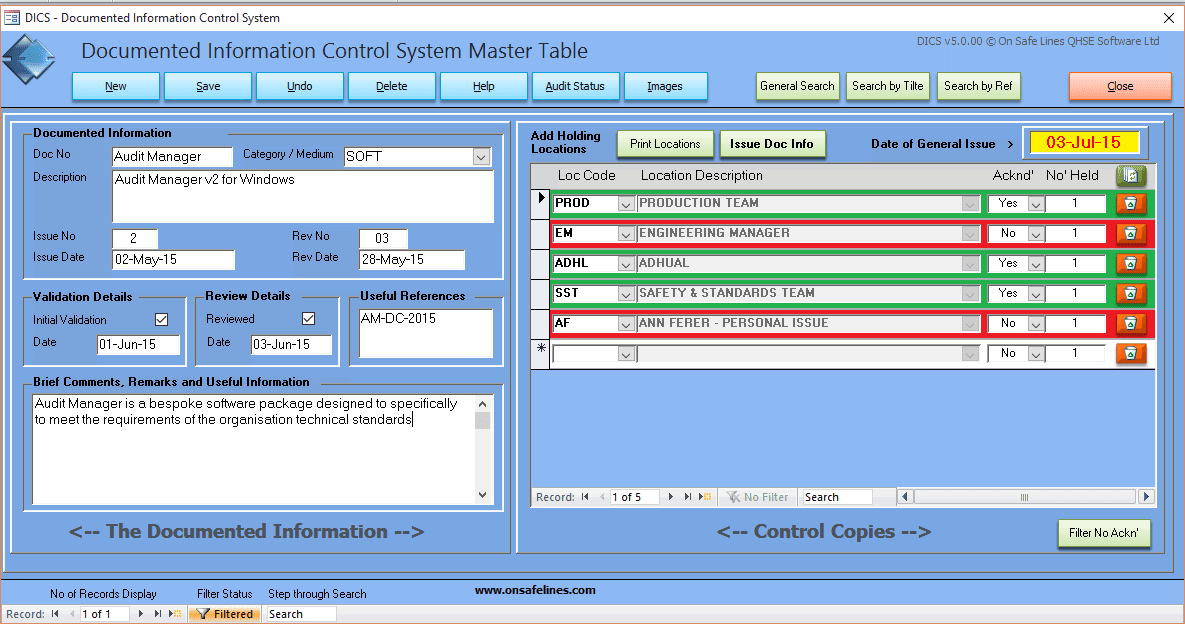
All of the requirements of this clause can be met using DICS - Document Issuing and Control system my On Safe Lines QHSE Software, see link below to learn more.
In the example below it shows a software program Audited Manager being controlled and issued to three locations.
continued on next page...
Useful integrated management system cross references
ISO 14001
•ISO 14001-2015 7.5 Documented information
ISO 45001
•ISO 45001-2018 7.5 Documented information
Help file v2.276.407 : QHSE Support - Website On Safe Lines
onsafelines.com QHSE Software 2025 : Webmaster: Brian G. Welch MSc(QHSE), NVQ4(OH&S), CMIOSH